Abstract
Introduction: Heavy menstrual bleeding (HMB) affects up to 51% of all people who menstruate and 93% of those with bleeding disorders. It is associated with iron deficiency and anemia (ID/IDA) and contributes to the global burden of morbidity and reduced health-related quality of life. First line therapy for HMB is oral tranexamic acid (TXA) and iron supplementation. Despite strong evidence to support the use of both therapies, and best efforts to counsel patients, outpatient adherence is often poor. In a retrospective practice audit between November, 2018 and January, 2019, adherence for TXA was 33%, and 40% to oral iron, respectively. A root-cause-analysis identified numerous barriers to adherence prompting the development of an interactive patient-directed website called "Take Control. Period.". The aim of the intervention was to improve adherence to TXA and iron through patient education and empowerment.
Methods: Research Ethics Board approval was obtained to study the use of "Take Control. Period." (www.takecontrolperiod.com) in outpatient hematology clinics at St. Michael's Hospital in Toronto, Canada for women with HMB and ID/A. All patients with HMB ≤ age 55 years who could benefit were provided with guest website access from February 1, 2020 onwards. The primary objective was to improve overall clinic medication adherence rate to target 80% for both TXA and iron as measured with a before and after. A retrospective review of patients' charts from November, 2018 to January, 2020 was used to provide baseline adherence data prior to intervention. Individual patient adherence was prospectively recorded at each clinic post-intervention. Before and after data was normally distributed with equal variances, therefore, compared using a two-sample t-test. Control chart analysis was completed using a standardized quality control evaluation for mean adherence rates before and after intervention implementation.
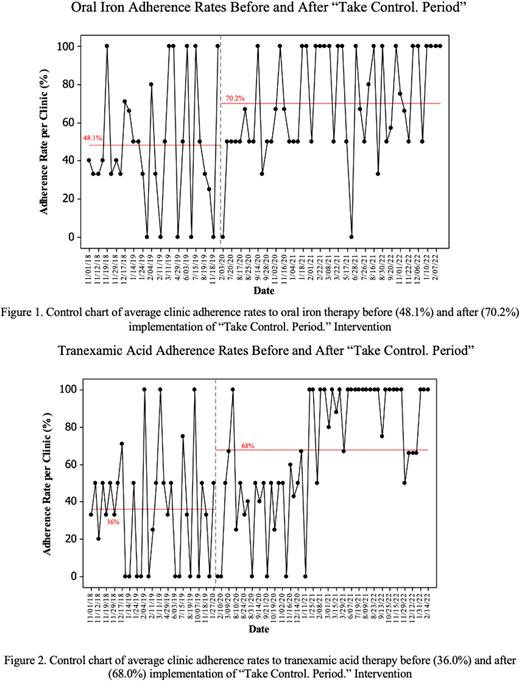
Results: A total of 90 clinical encounters with patients 55 with HMB on iron, and/or TXA were reviewed in the pre-intervention period. The average adherence rate per clinic was 48.1% for oral iron and 36.0% for oral TXA. Post-intervention, there were 238 similar clinical encounters. The average adherence rate per clinic post-intervention increased to 70.2% for iron (32.0% improvement, 95% CI 18.0-46.0%, p<0.001) (Figure 1), and 68.0% (22.1% improvement, 95% CI 7.6-36.6%, p=0.003) for TXA (Figure 2).
Conclusions: We developed a patient-centered interactive website for those with HMB that significantly improved adherence to TXA and iron, despite not meeting our original adherence targets. We are currently optimizing the website based on patient feedback and plan on making it globally accessible, free-of-charge, to diminish the burden and negative consequences of HMB.
Disclosures
James:Band Therapeutics: Consultancy; Bayer: Research Funding. Sholzberg:Octapharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.